Introduction Most red blood cell (RBC) alloimmunization in sickle cell disease (SCD) is caused by sensitization to Rh (D, C, c, E, and e) and K antigens. Provision of serologic Rh and K-matched RBCs has reduced but not eliminated Rh alloantibody formation due to the high genetic variation within the RH loci in Blacks. Over 85% of patients with SCD, as well as Black donors needed to support CEK matching programs, have variant RHD or RHCE alleles that result in loss or alteration of Rh antigens. These variant Rh proteins are not detected by standard serologic tests, and thus, genetically matched RBCs may be necessary to prevent all Rh alloimmunization events. We previously used bioinformatic modeling to show that RH genetic matching was achievable for a cohort of ~200 patients of whom ~50% were chronically transfused, and represented 98 different RHD/RHCE allele combinations, with only 10 additional donations each weekday (~1050 Black donors/month) than required for serologic Rh and K matching. The real-world feasibility of providing RH genotype-matched RBCs to patients requiring regular transfusion has not been tested.
Methods We performed a pilot study to evaluate the feasibility of providing RH genotype-matched RBCs from Black and Hispanic donors to non-Rh- and Rh-alloimmunized chronically transfused patients with SCD (1 to 18 years old). Black donor units at the blood center were first screened by serology, HEA BeadChip, and in-house RH SNP assays, and those selected for transfusion to study subjects had comprehensive RHD and RHCE genotyping. Data captured included RH genotypes of matched units identified, transfusion delays, unit age at time of transfusion, and pre-transfusion hemoglobin (Hb) and HbS levels. On-study values were compared to pre-study values obtained in the 6 to 12 transfusion visits prior to study enrollment.
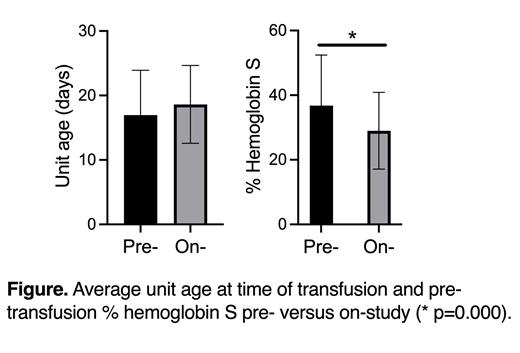
Results The study opened in February 2020. Due to the COVID-19 pandemic, donor center collections dropped to a nadir of ~500 Black donor units/month in 2020 but returned to ~1500 units/month currently, similar to pre-COVID numbers. For 14 participants (13 Black, 1 Hispanic), we transfused 327 RH genotype-matched units; 287 (88%) were exact RH genotype-matched (donor with identical genotype, homozygous for a matching RH haplotype, or RH allele match) and 40 (12%) were RH matched with the provision that RHD*RHD and *DAU0 and RHCE*ce and *ce48C are equivalent since no epitopes appear to differ between these alleles. No transfusion visits (n=183) were postponed due to the inability to identify RH genotype-matched units, including during the pandemic and national blood shortages that had a disproportionate impact on Black donations. Using individual patients as factors, ANOVA showed no statistically significant difference in unit age (days, Figure) or pre-transfusion Hb values between pre- and on-study transfusion visits. Although the goal maintenance pre-transfusion HbS levels varied between 30 and 50%, a statistically significant lower mean HbS on- (n=183) vs pre-study (n=139) (ANOVA F-ratio 15.4, p=0.000) (Figure) potentially suggests improved transfused RBC survival with RH genotype matching. This feasibility trial was not powered to detect a difference in alloantibody formation, but no Rh nor non-Rh antibody have been detected.
Conclusion Recruitment of minority blood donors is a growing initiative nationally needed to support CEK matching. Our preliminary results suggest that genetic RBC matching at the RH loci may be feasible with Black and Hispanic donors. Donor center RH genotyping can facilitate transfusion of RH genotype-matched RBCs, improve allocation of minority and rare donor units and, at the same time, potentially improve patient care by avoiding Rh alloimmunization for individuals with SCD.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal